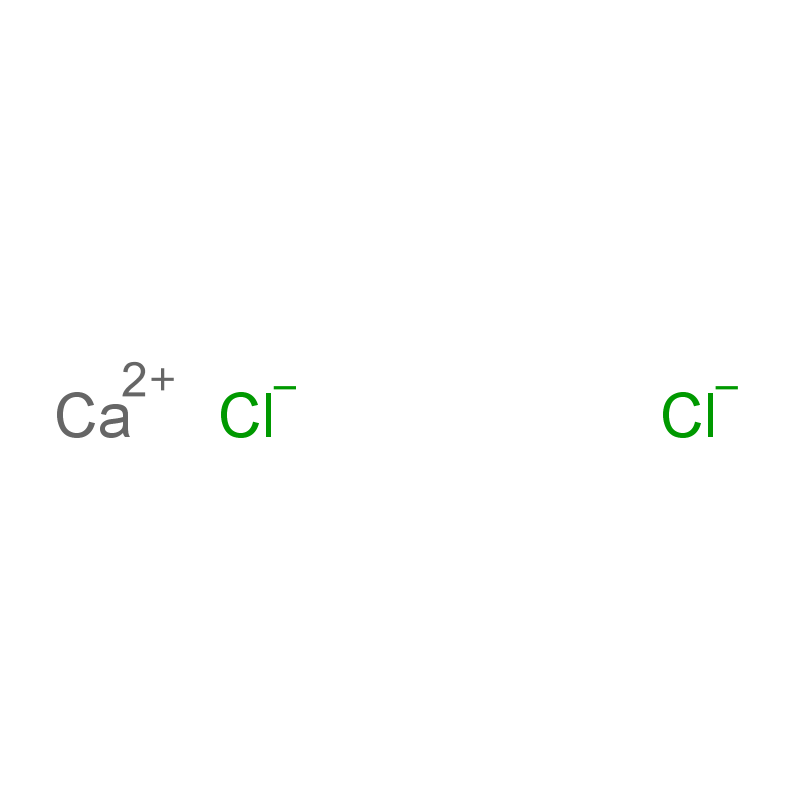
Products Description of Calcium chlorideCAS#10043-52-4
Anhydrous calcium chloride appears as a white, porous frit or granular solid and is highly deliquescent. It has a melting point of 782°C, a density of 2.15 g/cm³, and a boiling point exceeding 1600°C. It dissolves easily in water, releasing large amounts of heat, and is also soluble in ethanol and acetone.
The most common form is calcium chloride hexahydrate (CaCl₂·6H₂O), which consists of colorless trigonal crystals. This hexahydrate is also deliquescent, with a bitter and salty taste and a density of 1.71 g/cm³. At 29.92°C, it dissolves in its own crystal water (source: Chemicalbook). When heated to 30°C, it loses four water molecules to form calcium chloride dihydrate (CaCl₂·2H₂O)—a white, porous, and hygroscopic solid. Further heating can convert it into the monohydrate form. When the temperature exceeds 200°C, it loses all water molecules completely, forming anhydrous calcium chloride with strong hygroscopicity. Additionally, calcium chloride reacts with ammonia to produce the ammonia complex CaCl₂·8NH₃.
Parameters
Melting point | 772 °C (lit.) |
Boiling point | 1935 °C/1 atm (lit.) |
density | 1.086 g/mL at 20 °C |
vapor pressure | 0.01 mm Hg ( 20 °C) |
refractive index | n20/D 1.358 |
Fp | >1600°C |
storage temp. | Store at +5°C to +30°C. |
solubility | H2O: soluble |
form | |
color | White to gray |
Specific Gravity | 2.15 |
Odor | Odorless |
Flame Color | Redish |
PH | 8-10 (100g/l, H2O, 20℃) |
Water Solubility | 740 g/L (20 ºC) |
Sensitive | Hygroscopic |
λmax | λ: 260 nm Amax: 0.04 |
Crystal Structure | CaCl2 type |
crystal system | Nogata |
Merck | 14,1659 |
Space group | Pnnm |
Lattice constat | a/nmb/nmc/nmα/oβ/oγ/oV/nm30.6240.6430.429090900.1685 |
Stability: | Stable. Incompatible with zinc, water, strong acids, methyl vinyl ether, bromine trifluoride, boron oxide, calcium oxide. Hygroscopic. |
InChIKey | UXVMQQNJUSDDNG-UHFFFAOYSA-L |
CAS DataBase Reference | 10043-52-4(CAS DataBase Reference) |
NIST Chemistry Reference | Calcium dichloride(10043-52-4) |
EPA Substance Registry System | Calcium chloride (10043-52-4) |
Product Application of Calcium chlorideCAS#10043-52-4
Calcium chloride has been associated with tumorigenic and mutagenic effects, with human data supporting these properties; it may also interfere with hormonal balance.
Its applications are diverse: it is used as road salt for snow melting, a drying agent in desiccators, a dehydrating agent for organic liquids and gases, and an ingredient in refrigeration brines and antifreeze. Additionally, it serves as a dust-proofing agent, a food additive, a concrete hardening accelerator, and more.
Regarding incompatibilities and hazards: Aqueous solutions of calcium chloride are weakly basic. In the presence of moisture, it reacts with zinc to produce highly flammable hydrogen gas. It dissolves violently in water, releasing substantial heat. It is incompatible with substances including water, bromine trifluoride, 2-furan, and percarboxylic acids. Furthermore, in the presence of moisture, it may corrode certain building materials and metals.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days