Products Description of Formic Acid CAS# 64-18-6
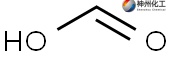
Formic acid, with the formula HCO₂H and alternative name methanoic acid, holds the distinction of being the simplest carboxylic acid. Its historical isolation involved the distillation of ant tissues, and the term "formic" is derived from "formica," the Latin term for "ant." Per IUPAC nomenclature standards, its official name is now methanoic acid. For industrial synthesis, the process involves treating carbon monoxide with an alcohol (e.g., methanol, commonly referred to as methyl alcohol) under catalytic conditions to yield formic acid.
Parameters
Melting point | |
Boiling point | 100-101 °C (lit.) |
density | 1.22 g/mL at 25 °C (lit.) |
vapor density | 1.03 (vs air) |
vapor pressure | 52 mm Hg ( 37 °C) |
refractive index | n20/D 1.377 |
FEMA | 2487 | FORMIC ACID |
Fp | 133 °F |
storage temp. | 2-8°C |
solubility | H2O: soluble1g/10 mL, clear, colorless |
pka | 3.75(at 20℃) |
form | Liquid |
color | APHA: ≤15 |
Specific Gravity | 1.216 (20℃/20℃) |
PH | 3.47(1 mM solution);2.91(10 mM solution);2.38(100 mM solution); |
Odor | at 0.10 % in water. pungent vinegar formyl |
Odor Type | |
biological source | synthetic |
explosive limit | 12-38%(V) |
Water Solubility | MISCIBLE |
λmax | λ: 260 nm Amax: 0.03 |
Sensitive | Hygroscopic |
Merck | 14,4241 |
JECFA Number | 79 |
BRN | 1209246 |
Henry's Law Constant | At 25 °C: 95.2, 75.1, 39.3, 10.7, and 3.17 at pH values of 1.35, 3.09, 4.05, 4.99, and 6.21, respectively (Hakuta et al., 1977) |
Exposure limits | TLV-TWA 5 ppm (~9 mg/m3) (ACGIH, MSHA, OSHA, and NIOSH); IDLH 100 ppm (180 mg/m3) (NIOSH). |
Dielectric constant | 58.0(16℃) |
Stability: | Stable. Substances to be avoided include strong bases, strong oxidizing agents and powdered metals, furfuryl alcohol. Combustible. Hygroscopic. Pressure may build up in tightly closed bottles, so bottles should be opened carefully and vented periodically. |
InChIKey | BDAGIHXWWSANSR-UHFFFAOYSA-N |
LogP | -0.540 |
CAS DataBase Reference | 64-18-6(CAS DataBase Reference) |
NIST Chemistry Reference | Formic acid(64-18-6) |
EPA Substance Registry System | Formic acid (64-18-6) |
Safety Information
Hazard Codes | T,C,Xi |
Risk Statements | 23/24/25-34-40-43-35-36/38-10 |
Safety Statements | 36/37-45-26-23-36/37/39 |
RIDADR | UN 1198 3/PG 3 |
OEB | B |
OEL | TWA: 5 ppm (9 mg/m3) |
WGK Germany | 2 |
RTECS | LP8925000 |
F | 10 |
Autoignition Temperature | 1004 °F |
TSCA | Yes |
HazardClass | 8 |
PackingGroup | II |
HS Code | 29151100 |
Hazardous Substances Data | 64-18-6(Hazardous Substances Data) |
Toxicity | LD50 in mice (mg/kg): 1100 orally; 145 i.v. (Malorny) |
IDLA | 30 ppm |
Product Application of Formic Acid CAS# 64-18-6
Formic acid has both natural occurrence and industrial utility. Naturally, it is found in the sting secretions of ants and bees. Industrially, its uses span various sectors: it serves as a raw material for manufacturing esters and salts; in textile and paper industries, it aids in dyeing and finishing processes to enhance product quality; it is applied in electroplating operations to improve coating performance; in leather production, it participates in leather treatment steps; and it helps coagulate rubber latex in rubber processing. Additionally, it acts as a reducing agent in relevant chemical reactions.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days